Novel strategies for enhancing antitumor activity and persistence to reduce CAR T cell methylation l
DNA methylation refers to the methylation of specific bases on the DNA sequence at C-5 sites of cytosine, N-6 sites of adenine and G-7 sites of guanine under the catalysis of DNA methyltransferase (DNMTs). In general, DNA methylation adds methyl groups to 5 '-C of cytosine residues in CpG dinucleotides [1].
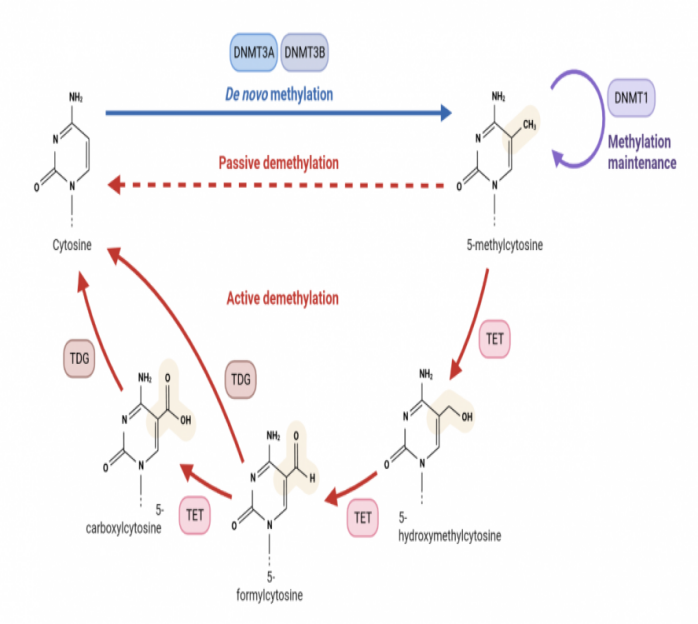
Figure 1 Schematic diagram of DNA methylation and demethylation (photo by biorender)
DNA methylation in eukaryotes is an important epigenetic modification that regulates gene expression and is critical in embryonic development. It involves many aspects such as genetic material stabilization, gene expression regulation, X chromosome inactivation, transposon silencing, histone modification and so on. DNA methylation, if not corrected during cell division, can induce genetic diseases or cancer, and the way organisms methylate is stable and heritable. Therefore, dysmethylation of DNA is closely related to human diseases, such as autoimmune diseases, metabolic disorders, nervous system diseases and cancer [2].
DNA methylation changes include hypermethylation and hypomethylation, among which DNA hypermethylation is a potential biological marker for early diagnosis and prognosis of tumors. When hypermethylation occurs, it often leads to the disorder of cell cycle regulation and transcriptional silencing such as DNA repair genes, resulting in the inactivation of many cell pathways, affecting the growth regulation and differentiation of normal cells, and then leading to the occurrence of tumors. For example, p16INK4a gene promoter hypermethylation is present in a variety of solid tumors, such as liver, lung, pancreatic, breast, cervical, or bladder cancers, as well as melanoma and glioma. The overexpression of p16INK4a is involved in cell senescence, senescence and cell cycle process. This protein blocks cell cycle progression by inhibiting cyclin-dependent kinases 4 and 6, which mediate the phosphorylation of retinoblastoma protein (pRB) in G1 phase, thereby blocking cell cycle progression. DNA hypermethylation leads to increased p16INK4 silencing, leading to increased levels of pRB phosphorylation, which leads to infinite cell proliferation [3]. The hypomethylation effect leads to increased DNA damage, chromatin desagglutination and chromosomal instability. In addition, DNA hypomethylation can activate genes that are silenced by hypermethylated promoters. For example, the gene from MAGEA is expressed only in spermatogonocytes and not in other somatic tissues. However, when their promoters undergo demethylation, they can be expressed in a variety of cancers. Abnormal expression of MAGEA genes is often associated with increased aggressiveness and malignancy of breast, lung, and colorectal tumors [4].
The change of DNA methylation level can promote the development of tumor, so DNA demethylation has become an important direction of tumor therapy. At present, the most commonly used treatment strategy is to develop small molecule drugs to regulate the activity of DNMT, a key DNA methylation enzyme. That is, cytosine analogues are designed for cytosine, the substrate of DNMT, which can covalently bind to DNMT, so that DNMT cannot detach from chromatin, thus inhibiting its activity. There are currently two cytosine analogues on the market, azacytidine and decitabine. Azacytidine is a pyrimidine nucleoside analogue of cytidine. After cellular uptake and enzymatic bioconversion to triphosphate nucleotide, azacytidine is incorporated into DNA and RNA to slow down the disease process by inhibiting DNA/RNA methyltransferase, DNA synthesis and cell proliferation. Azacytidine is mainly used as an antitumor drug, and is clinically used in the treatment of breast cancer, bowel cancer and melanoma. It is indicated for adults with acute myeloid leukemia who have a first complete response (CR) after intensive induction chemotherapy or a first complete response but whose blood count has not fully recovered (CRi) and cannot continue to complete intensive therapy. Decitabine, a natural adenosine analogue of 2 '-deoxycytidylate, reduces DNA methylation by inhibiting DNA methyltransferase, thereby inhibiting tumor cell proliferation and preventing the development of drug resistance. Decitabine is the strongest known specific inhibitor of DNA methylation and belongs to the S-phase cell cycle specific drug. Applicable to patients with intermediate-risk 1, intermediate-risk 2, and high-risk primary and retreated myelodysplastic syndromes (MDS), including primary and secondary MDS, according to FAB subtypes: Refractory anemia, refractory anemia with ring siderocytosis, refractory anemia with protocytosis, refractory anemia with protocytosis - transformation, chronic granulomonocytic leukemia.
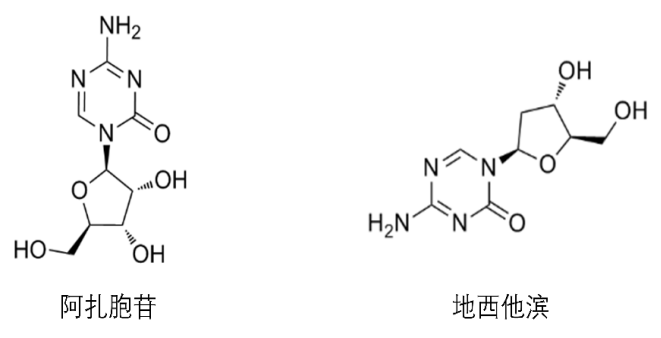

Figure 1 Schematic diagram of DNA methylation and demethylation (photo by biorender)
DNA methylation in eukaryotes is an important epigenetic modification that regulates gene expression and is critical in embryonic development. It involves many aspects such as genetic material stabilization, gene expression regulation, X chromosome inactivation, transposon silencing, histone modification and so on. DNA methylation, if not corrected during cell division, can induce genetic diseases or cancer, and the way organisms methylate is stable and heritable. Therefore, dysmethylation of DNA is closely related to human diseases, such as autoimmune diseases, metabolic disorders, nervous system diseases and cancer [2].
DNA methylation changes include hypermethylation and hypomethylation, among which DNA hypermethylation is a potential biological marker for early diagnosis and prognosis of tumors. When hypermethylation occurs, it often leads to the disorder of cell cycle regulation and transcriptional silencing such as DNA repair genes, resulting in the inactivation of many cell pathways, affecting the growth regulation and differentiation of normal cells, and then leading to the occurrence of tumors. For example, p16INK4a gene promoter hypermethylation is present in a variety of solid tumors, such as liver, lung, pancreatic, breast, cervical, or bladder cancers, as well as melanoma and glioma. The overexpression of p16INK4a is involved in cell senescence, senescence and cell cycle process. This protein blocks cell cycle progression by inhibiting cyclin-dependent kinases 4 and 6, which mediate the phosphorylation of retinoblastoma protein (pRB) in G1 phase, thereby blocking cell cycle progression. DNA hypermethylation leads to increased p16INK4 silencing, leading to increased levels of pRB phosphorylation, which leads to infinite cell proliferation [3]. The hypomethylation effect leads to increased DNA damage, chromatin desagglutination and chromosomal instability. In addition, DNA hypomethylation can activate genes that are silenced by hypermethylated promoters. For example, the gene from MAGEA is expressed only in spermatogonocytes and not in other somatic tissues. However, when their promoters undergo demethylation, they can be expressed in a variety of cancers. Abnormal expression of MAGEA genes is often associated with increased aggressiveness and malignancy of breast, lung, and colorectal tumors [4].
The change of DNA methylation level can promote the development of tumor, so DNA demethylation has become an important direction of tumor therapy. At present, the most commonly used treatment strategy is to develop small molecule drugs to regulate the activity of DNMT, a key DNA methylation enzyme. That is, cytosine analogues are designed for cytosine, the substrate of DNMT, which can covalently bind to DNMT, so that DNMT cannot detach from chromatin, thus inhibiting its activity. There are currently two cytosine analogues on the market, azacytidine and decitabine. Azacytidine is a pyrimidine nucleoside analogue of cytidine. After cellular uptake and enzymatic bioconversion to triphosphate nucleotide, azacytidine is incorporated into DNA and RNA to slow down the disease process by inhibiting DNA/RNA methyltransferase, DNA synthesis and cell proliferation. Azacytidine is mainly used as an antitumor drug, and is clinically used in the treatment of breast cancer, bowel cancer and melanoma. It is indicated for adults with acute myeloid leukemia who have a first complete response (CR) after intensive induction chemotherapy or a first complete response but whose blood count has not fully recovered (CRi) and cannot continue to complete intensive therapy. Decitabine, a natural adenosine analogue of 2 '-deoxycytidylate, reduces DNA methylation by inhibiting DNA methyltransferase, thereby inhibiting tumor cell proliferation and preventing the development of drug resistance. Decitabine is the strongest known specific inhibitor of DNA methylation and belongs to the S-phase cell cycle specific drug. Applicable to patients with intermediate-risk 1, intermediate-risk 2, and high-risk primary and retreated myelodysplastic syndromes (MDS), including primary and secondary MDS, according to FAB subtypes: Refractory anemia, refractory anemia with ring siderocytosis, refractory anemia with protocytosis, refractory anemia with protocytosis - transformation, chronic granulomonocytic leukemia.

Figure 2 Structural formula of azacytidine and decitabine
CAR-T therapy is one of the most important new advances in immunotherapy targeting cancer and has produced many breakthroughs in hematologic tumors, but CAR-T therapy is often limited by T cell depletion. Clinically, analysis of biopsy samples during treatment or after relapse will help determine the extent to which CAR-T cell failure has led to a loss of disease control in patients. Transfusions of CD19-targeted, 4-1BB-based CAR T cells into B-ALL patients obtained DNA methylation signatures associated with failure after 4 weeks. Another study showed that TIM3+PD-1+ CAR T cells persisted in the blood of patients with chronic lymphocytic leukemia several months after CAR-T infusion, and these cells were enriched in patients with poor clinical outcomes [5]. If CAR T cells are indeed damaged by failure, interrupting this underfunctioning cell state could improve the therapeutic effectiveness of the disease. The team of Christopher J. Gamper and Kenneth R. Cooke at Sidney Meyer Comprehensive Cancer Center of Johns Hopkins University in the United States found that DNA methyltransferase 3a (DNMT3a) is an important part of the epigenetic mechanism that stabilizes the response of active T cells [6]. Studies have found that knocking out DNMT3a gene of CAR-T cells can prevent T cell failure and enhance anti-tumor activity [7]. At the same time, the anti-tumor activity, cytokine production and proliferation of decitabine-treated CAR T cells were enhanced in vitro and in vivo studies. In addition, CAR T cells after treatment can clear large tumors at low doses and establish an effective memory response when tumors reattack [8]. These results indicate that DNMT3a can be used as a universal target to improve the efficacy of CAR-T therapy, and the combination of DNMT3A with DNMT3A provides a new way to develop more effective CAR-T cell therapy.
References:
[1]Schübeler Dirk. ESCI award lecture: regulation, function and biomarker potential of DNA methylation. European journal of clinical investigation. 2015 Mar;45(3):288-93.
[2]Martisova Andrea, et al. DNA Methylation in Solid Tumors: Functions and Methods of Detection. International journal of molecular sciences. 2021 Apr 19;22(8):4247.
[3]Junan Li , et al. Regulatory mechanisms of tumor suppressor P16(INK4A) and their relevance to cancer. Biochemistry. 2011 Jun 28;50(25):5566-82.
[4]Poojary Manish, et al. Prognostic Value of Melanoma-Associated Antigen-A (MAGE-A) Gene Expression in Various Human Cancers: A Systematic Review and Meta-analysis of 7428 Patients and 44 Studies. Molecular diagnosis & therapy. 2020 Oct;24(5):537-555.
[5]Chow Andrew, et al. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022 Dec;19(12):775-790.
[6]Ktena YP, et al. Donor T cell DNMT3a regulates alloreactivity in mouse models of hematopoietic stem cell transplantation. J Clin Invest. 2022 Jul 1;132(13):e158047.
[7]Prinzing Brooke, et al. “Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med. 2021 Nov 17;13(620):eabh0272.
[8]Wang Yao, et al. Low-dose decitabine priming endows CAR T cells with
enhanced and persistent antitumour potential via epigenetic reprogramming. Nat Commun. 2021 Jan 18;12(1):409.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies, so that more people understand the new development of biomedicine. The content of this article is only used for information exchange, and the platform remains neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information in this article should not be used as a diagnosis or treatment, is not a substitute for professional medical advice, and the company's website will not assume any responsibility. The final interpretation of the content of the above statement belongs to the company's website, this statement will apply to the company's website all the time to share the article, thank you for your cooperation! Copyright description: The copyright of the article belongs to Shenzhen Cell Valley, individuals are welcome to forward to the circle of friends, media or institutions without authorization, reproduced in any form to other platforms, will be regarded as infringement. For reprinting, please contact email: contact@duanglink.com
References:
[1]Schübeler Dirk. ESCI award lecture: regulation, function and biomarker potential of DNA methylation. European journal of clinical investigation. 2015 Mar;45(3):288-93.
[2]Martisova Andrea, et al. DNA Methylation in Solid Tumors: Functions and Methods of Detection. International journal of molecular sciences. 2021 Apr 19;22(8):4247.
[3]Junan Li , et al. Regulatory mechanisms of tumor suppressor P16(INK4A) and their relevance to cancer. Biochemistry. 2011 Jun 28;50(25):5566-82.
[4]Poojary Manish, et al. Prognostic Value of Melanoma-Associated Antigen-A (MAGE-A) Gene Expression in Various Human Cancers: A Systematic Review and Meta-analysis of 7428 Patients and 44 Studies. Molecular diagnosis & therapy. 2020 Oct;24(5):537-555.
[5]Chow Andrew, et al. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022 Dec;19(12):775-790.
[6]Ktena YP, et al. Donor T cell DNMT3a regulates alloreactivity in mouse models of hematopoietic stem cell transplantation. J Clin Invest. 2022 Jul 1;132(13):e158047.
[7]Prinzing Brooke, et al. “Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med. 2021 Nov 17;13(620):eabh0272.
[8]Wang Yao, et al. Low-dose decitabine priming endows CAR T cells with
enhanced and persistent antitumour potential via epigenetic reprogramming. Nat Commun. 2021 Jan 18;12(1):409.
Disclaimer: Shenzhen Cell Valley is committed to the research of cell and gene therapy, in order to promote emerging technologies, so that more people understand the new development of biomedicine. The content of this article is only used for information exchange, and the platform remains neutral on the content, statements and opinions of the article, and does not represent the position and views of Shenzhen Cell Valley. The relevant information in this article should not be used as a diagnosis or treatment, is not a substitute for professional medical advice, and the company's website will not assume any responsibility. The final interpretation of the content of the above statement belongs to the company's website, this statement will apply to the company's website all the time to share the article, thank you for your cooperation! Copyright description: The copyright of the article belongs to Shenzhen Cell Valley, individuals are welcome to forward to the circle of friends, media or institutions without authorization, reproduced in any form to other platforms, will be regarded as infringement. For reprinting, please contact email: contact@duanglink.com
Previous:Metabolic challenges in CAR-T cell therapy
Next:Nothing


